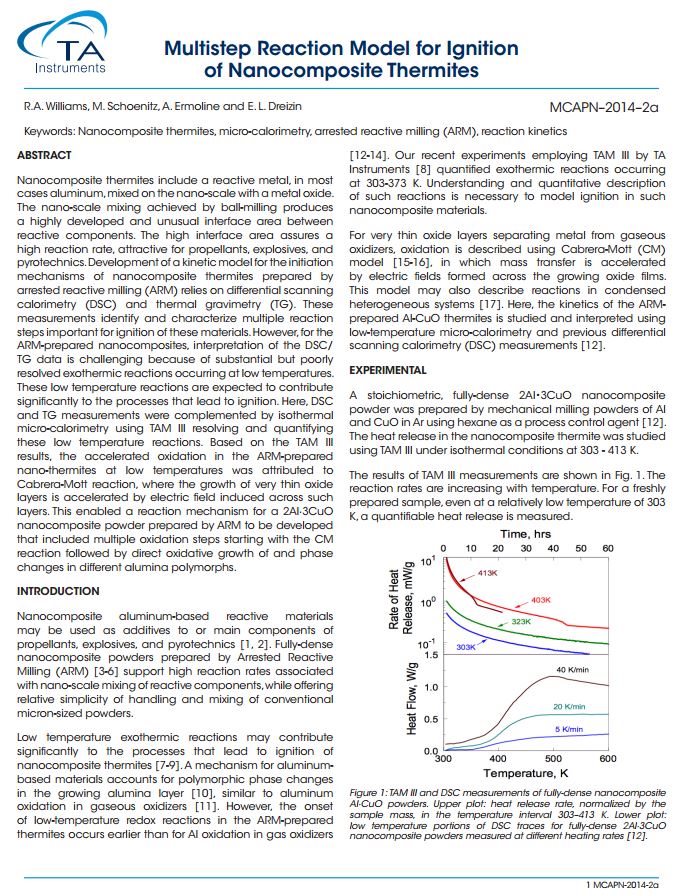
ABSTRACT Nanocomposite thermites include a reactive metal, in most cases aluminum, mixed on the nano-scale with a metal oxide. The nano-scale mixing achieved by ball-milling produces a highly developed and unusual interface area between reactive components. The high interface area assures a high reaction rate, attractive for propellants, explosives, and pyrotechnics. Development of a kinetic model for the initiation mechanisms of nanocomposite thermites prepared by arrested reactive milling (ARM) relies on differential scanning calorimetry (DSC) and thermal gravimetry (TG). These measurements identify and characterize multiple reaction steps important for ignition of these materials. However, for the ARM-prepared nanocomposites, interpretation of the DSC/ TG data is challenging because of substantial but poorly resolved exothermic reactions occurring at low temperatures. These low temperature reactions are expected to contribute significantly to the processes that lead to ignition. Here, DSC and TG measurements were complemented by isothermal micro-calorimetry using TAM III resolving and quantifying these low temperature reactions. Based on the TAM III results, the accelerated oxidation in the ARM-prepared nano-thermites at low temperatures was attributed to Cabrera-Mott reaction, where the growth of very thin oxide layers is accelerated by electric field induced across such layers. This enabled a reaction mechanism for a 2Al∙3CuO nanocomposite powder prepared by ARM to be developed that included multiple oxidation steps starting with the CM reaction followed by direct oxidative growth of and phase changes in different alumina polymorphs.
Keywords: Nanocomposite thermites, micro-calorimetry, arrested reactive milling (ARM), reaction kinetics MCAPN–2014–2a
R.A. Williams, M. Schoenitz, A. Ermoline and E. L. Dreizin
