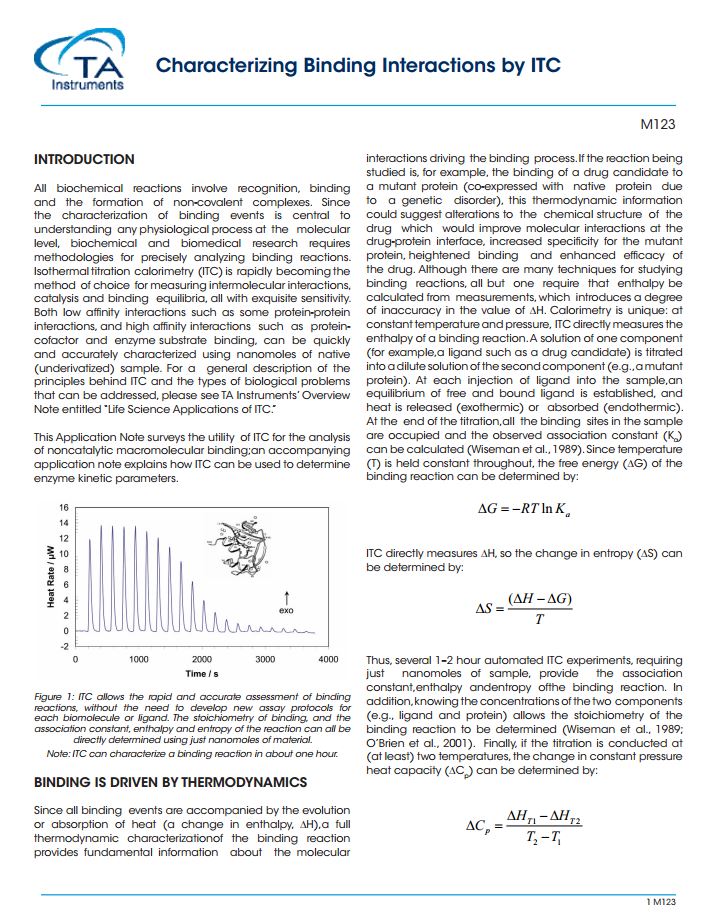
INTRODUCTION All biochemical reactions involve recognition, binding and the formation of non-covalent complexes. Since the characterization of binding events is central to understanding any physiological process at the molecular level, biochemical and biomedical research requires methodologies for precisely analyzing binding reactions. Isothermal titration calorimetry (lTC) is rapidly becoming the method of choice for measuring intermolecular interactions, catalysis and binding equilibria, all with exquisite sensitivity. Both low affinity interactions such as some protein-protein interactions, and high affinity interactions such as proteincofactor and enzyme substrate binding, can be quickly and accurately characterized using nanomoles of native (underivatized) sample. For a general description of the principles behind lTC and the types of biological problems that can be addressed, please see TA Instruments’ Overview Note entitled “Life Science Applications of lTC.” This Application Note surveys the utility of lTC for the analysis of noncatalytic macromolecular binding;an accompanying application note explains how lTC can be used to determine enzyme kinetic parameters.
Keywords: M123
